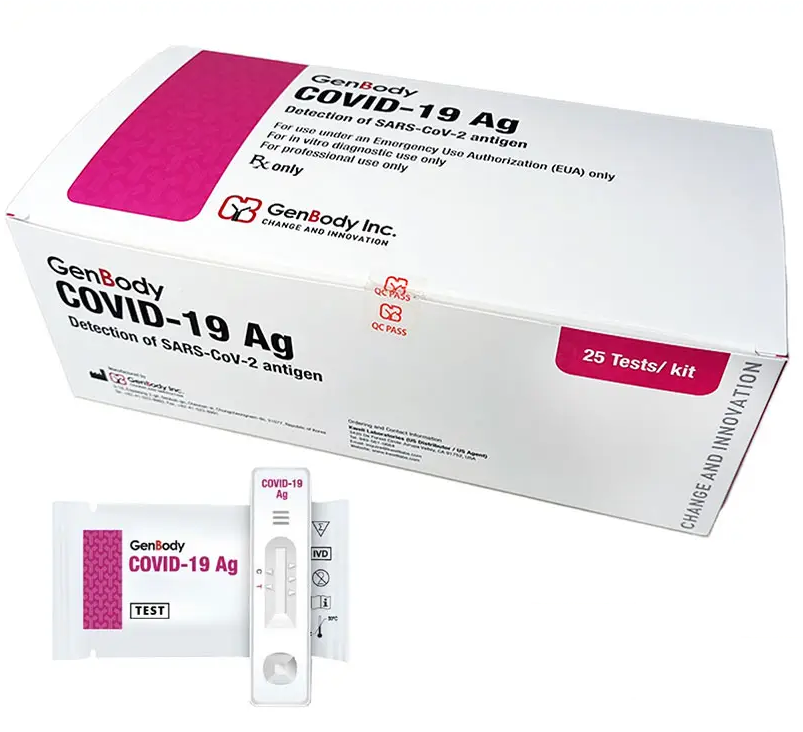
Description
The GenBody COVID-19 Ag is an immunochromatographic rapid diagnostic test (RDT) intended for the qualitative
detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) or anterior nasal (AN)
swab specimens from individuals who are suspected of COVID-19 by their healthcare provider within the first six
days of symptom onset. Testing is limited to laboratories certified under the Clinical Laboratory Improvement
Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance or Certificate of Accreditation
· This product has not been FDA cleared or approved, but has been authorized by FDA
under an EUA;
· This product has been authorized only for the detection of proteins from SARS-
CoV-2, not for any other viruses or pathogens; and,
· The emergency use of this product is only authorized for the duration of the
declaration that circumstances exist justifying the authorization of emergency use
of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section
564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-
3(b)(1), unless the declaration is terminated or authorization is revoked sooner








Reviews
There are no reviews yet.